T-E Meds website: https://www.temeds.com/
CHO-TEM ADCs, resulting from a strategic collaboration between CHO Pharma and T-E Meds, represent a series of ADCs.
These constituted antibodies encompass whole IgG molecules, targeting well-established carcinogenic antigens and are site-specifically conjugated to four drug bundles, each carrying 2-4 cytotoxic molecules.
Notably, CHO-TEM ADCs exhibit homogeneity with high DAR of 8, 12 or 16, showing superior in vitro and in vivo stabilities.
The molecular structure of CHO-TEM ADCs are characterized by the conjugation of Trastuzumab (TRZ), a humanized anti-HER2 IgG monoclonal antibody, with drug bundles containing distinct drug combinations: TRZ-8MMAF (1TRZ is conjugated to 8 molecules of monomethyl auristatin F, MMAF), TRZ-8DXd (1TRZ is conjugated to 8 molecules of exatecan derivative, DXd) and TRZ-4(MMAF+DXd) (1TRZ is conjugated to 4 molecules each of MMAF and DXd).
All of these CHO-TEM ADCs are produced with purities exceeding 95%.
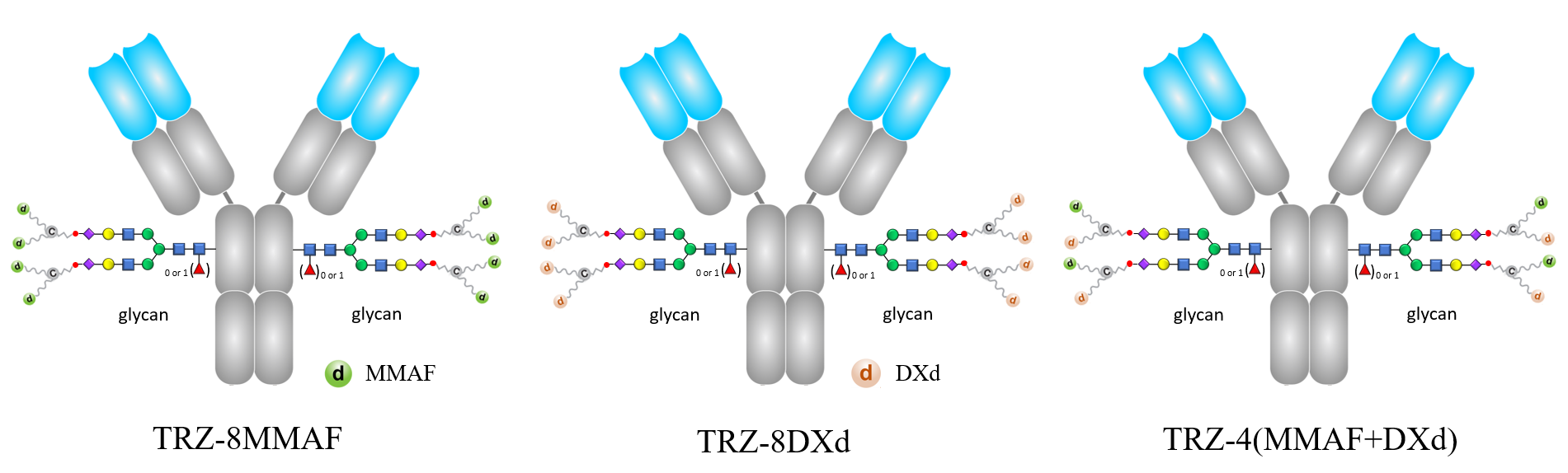
The molecular structures of CHO-TEM ADCs.
The % of cell viabilities for SKBR3 breast cancer cells after 5 days of incubation with TRZ, TRZ-deruxtecan (Enhertuâ) and CHO-TEM ADCs are shown below as a function of drug concentration.
Cytotoxic effects were comparable between TRZ-8DXd and TRZ-deruxtecan, while TRZ-8MMAF and TRZ-4(MMAF+DXd) exhibited higher potency in killing SKBR3 cells.
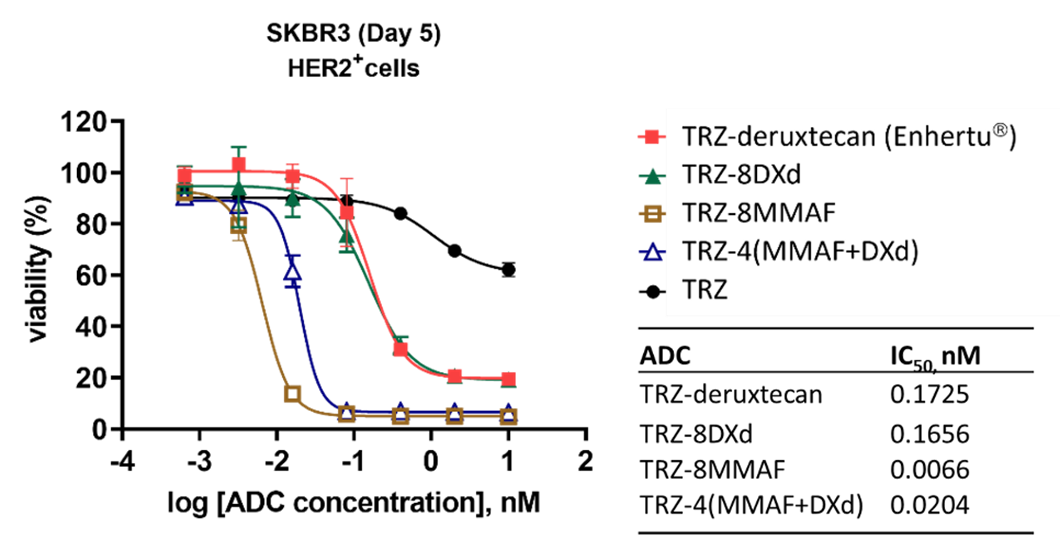
CHO-TEM ADCs showed high potent killing effects in SKBR3 breast cancer cells.

